Events

Low Level Light Therapy: the Path Forward
20-22 August 2014
OSA Headquarters, Washington, DC, USA
Low Level Light Therapy (LLLT) also known as photobiomodulation (PBM), a light therapy using visible and NIR radiation, is almost 50 years old. Hundreds of positive clinical trials and thousands of laboratory studies have been published yet LLLT/PBM has not been adopted by mainstream medicine. This meeting addressed the reasons for this failure and identified paths forward.
Background
LLLT/PBM is absorbed by endogenous chromophores catalysing non-thermal, non-cytotoxic, biological reactions. It has been shown in clinical trials and laboratory studies that LLLT / PBM has tissue regenerative effects (skin, tendon, muscle, bone, nerves, spinal cord and cerebral cortex). It has very good anti-inflammatory effects, analgesic effects; it improves function of healthy organs (brain, muscle) and also has cytoprotective effects (brain, muscle).
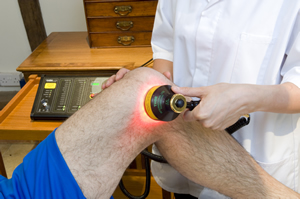
There are about 400 hundred controlled clinical trials and thousands of laboratory studies investigating the biological mechanisms, irradiation parameters and dose with good results seen in some of the world’s most challenging clinical conditions including: traumatic brain injury, stroke, spinal cord injury, peripheral nerve injuries, macular degeneration, heart disease, osteoarthritis, non-healing diabetic wounds, postherpetic neuralgia, as well as less challenging conditions such as sports injuries and skin conditions.
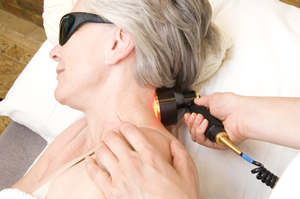
The clinical trials and laboratory studies consistently show good repeatable results where drugs and surgery are failing, yet LLLT has not been widely adopted by mainstream medicine. Why is this? The reason for this depends on who you ask.
According to:- Scientists it’s a: lack of mechanism, parameters and dose evidence
- Doctors it’s a: lack of large clinical trials / systematic reviews
- Hospital administrators it’s a: lack of reimbursement
- Insurance it’s a: lack of cost / benefit evidence
- Industry representatives it’s a: marketing hype / misinformation
- Development professionals it’s a: lack of a focused central tech/research/clinical corridor
- What is real and what is not?
- What is holding us back?
- What do we need to move forward?
READ THE INCUBATOR BLOG
Hosted by:
Juanita Anders
Uniformed Services University of the Health Sciences
Praveen Arany
National Institute of Health
James Carroll
THOR Photomedicine Ltd
Michael R Hamblin
Harvard Medical School
Donald E. Patthoff, DDS
