It takes blood, sweat, and SERS to image single cells
Ken Tichauer
Throw out those old dusty fluorescent molecules and welcome in the next generation of optical contrast agents. SERS (Surface Enhanced Raman Scattering/Spectroscopy) nanoparticles are sophisticated new contrast agents that offer some distinct advantages over conventional fluorescent molecules for investigating molecular biology.
Major advantage #1:
An unprecedented level of molecular sensitivity has been observed using SERS nanoparticles with the ability to detect a single cell in “wide-field” imaging. Imagine you are able to inject a SERS particle into a patient that is about to undergo cancer surgery. If that SERS particle is designed to “stick” only to cancer cells, and SERS nanoparticle imaging has the ability to highlight a single cell in a wide imaging field, then there is potential for a surgeon to image SERS after removing the bulk of the cancer to “see” if there are remaining tumor cells in the surgical field, down to the individual cell.
Major advantage #2:
SERS nanoparticles have incredibly distinct emission patterns, which allow multiple different “flavors” of particles to be imaged at the same time, using the same excitation light. The more, the merrier, right? What this allows is for many different types of biological molecules to be imaged in biological samples or subjects at the same time. All diseases are complex, particularly cancer, so being able to measure the expression of many disease pathways will offer a more complete understanding and more sensitive diagnosis of these diseases going forward.
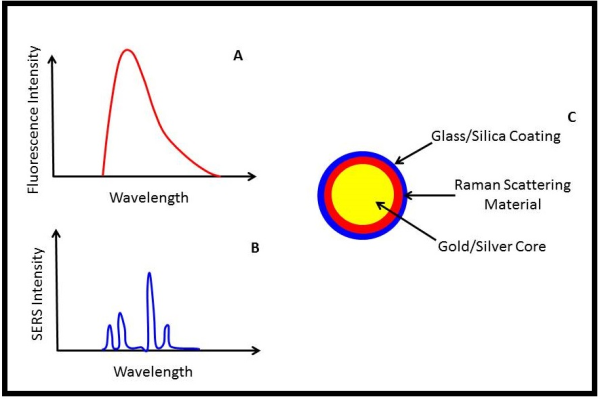
To understand where this incredible specificity and sensitivity comes from when imaging SERS nanoparticles, we can skip details of Raman scattering and how it is “enhanced”, and instead focus on what SERS emission looks like compared to fluorescence emission.
When a conventional fluorescence molecule is excited with the right wavelength of light, it emits fluorescence over a broad band of colors/wavelengths (see Fig A). It’s certainly possible to image multiple fluorescence molecules, and hence multiple biomolecular pathways, simultaneously by choosing fluorophores that emit light at distinctly different wavelengths; however, these different wavelengths of light can interact in very different ways with the surrounding tissue, making quantitative comparisons of fluorescence images difficult. Moreover, depending on the wavelength of light used for exciting fluorescence, considerable “background” fluorescence in tissue (known as autofluorescence) will be present, limiting the ultimate sensitivity of fluorescence detection.
The unique advantage of SERS nanoparticles is that they emit a much more distinct pattern of light than fluorescence when illuminated (Fig. B). This allows superior separation of SERS nanoparticle signal from background signals, which in turn allows single cells to be imaged1, and many different SERS nanoparticles to be imaged simultaneously2.
While clinical translation of SERS nanoparticles may be a ways off, they offer enormous potential for single cell tracking and multiple receptor imaging.
1. Kircher, M.F., et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nature medicine 18, 829-834 (2012).
2. Zavaleta, C.L., et al. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proceedings of the National Academy of Sciences of the United States of America 110, E2288-2297 (2013).
